RapidDx
Lab in Your Hand
The groundbreaking RapidDx device is designed to transform saliva-based diagnostics, providing quick initial assessments for diseases including Alzheimer’s and Parkinson’s and conditions like concussion.
Developed by a team
of scientific experts
Noninvasive,
revolutionary technology
Patented, handheld platform
for rapid results
Saliva as Medical Assessment Medium
Saliva is increasingly recognised as an excellent first-line option for noninvasive diagnostic assessments. Easily obtainable specimens can be used to detect many analyte types, including proteins, DNA, RNA, hormones, drugs, and others.
Our Patented
Rapid Assessment Device (RAD)

Saliva-based diagnostics for diseases of the ageing and traumatic brain injuries
Although saliva is painless to obtain, an effective and simple mechanism for the collection and testing of oral fluids is essential. Enter the patented Rapid Assessment Device (RAD) by RapidDx, designed to be a true game changer in diagnostic technology.
The RapidDx device has been described as a ‘lab in your hand’. The world became accustomed to using these types handheld products during the COVID-19 pandemic. However, compared to COVID-19 home tests, the RapidDx device will be less complicated. Here is how it will be used:
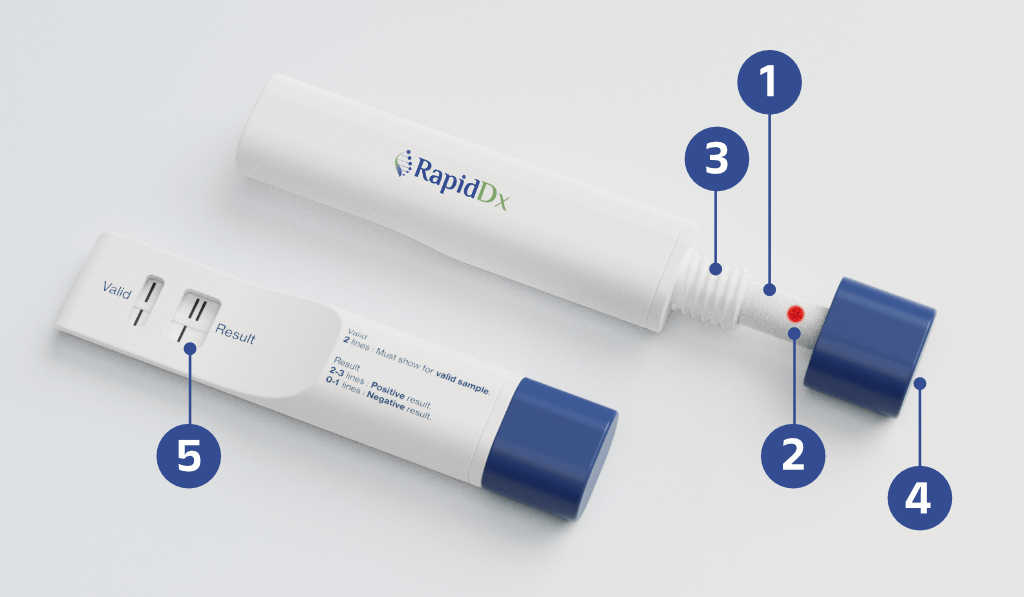
Step 1
User inserts saliva absorption pad into the cheek or sub-lingual pocket
Step 2
Red ‘indicator’ dot informs user sufficient saliva has been collected
Step 3
User inserts collection wick into the device
Step 4
User turns the knob, compressing saliva to the test strips
Step 5
Assessment devices for different conditions
The use of the device varies according to the disease or condition being tested. For example, the RapidDx RAD for concussion assessment is designed to be an over-the-counter (OTC) product kept in a first aid kit, a handbag, or a car to enable a rapid response to falls, vehicle accidents, sports injuries, and other traumatic events.
The RapidDx RADs for Alzheimer’s or Parkinson’s diseases will use a different portfolio of biomarkers that are specific for such diseases and quantitatively calculated in saliva specimens. In this case, a medical professional should explain the quantitative data to the patient, offer appropriate counseling, and recommend courses of action for both the patient and their caregivers if the test results are positive.